Singclean Human Chorionic Gonadotropin (HCG) Pregnancy Test Kit
(Colloidal Gold)
For Self Testing Specimens: Urine
Instructions For Use Specimens: Urine
IVD for Self-testing, for professional use, for near-patient testing
CE 0123
[INTENDED USE]
The human chorionic gonadotropin (HCG) pregnancy test kit is a rapid, one-step lateral flow immunoassay for the qualitative detection of human chorionic gonadotropin (HCG) in urine to aid in the detection of pregnancy.
This test is for women who are planning pregnancy or suspected pregnant to aid in the early detection of pregnancy. This test is suitable for self-testing, professional use and near-patient testing.
[INTRODUCTION]
Human chorionic gonadotropin (HCG) is a glycoprotein secreted by trophoblast cells of the placenta and consists of alpha and beta dimerized glycoproteins, which is proliferated rapidly in the first 8 weeks of pregnancy to maintain pregnancy. After about 8 weeks of gestation, HCG gradually declines until it reaches a relatively stable level at about 20 weeks.
Using the HCG antibody to qualitatively detect HCG in the urine of pregnant women can quickly obtain the results in the early stage of pregnancy, which is an effective method for auxiliary diagnosis
[PRINCIPLE]
The Human Chorionic Gonadotropin (HCG) Pregnancy test kit is a colloidal gold immunochromatographic assay.
The test uses α-HCG-mAb (test line T) and goat anti-mouse lgG (control line C) immobilised on a nitrocellulose strip. Theburgundy colored coniugate pad contains colloidal gold coniugated to β-HCG-mAb. When sample is added to the sample well, HCG will combine with the β-HCG-mAb conjugate to form an antigen-antibody complex, This complex migrates through nitrocellulose membrane by capillary action, When the complex meets the line of the α-HCG-mAb (test line T), the complex is trapped forming a burgundy colored band which confirms a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
The test contains an internal control (C band) which should exhibit a burgundy colored band regardless of the color development any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
[MATERIALS PROVIDED]
Test strip/ Test cassette/ Test midstream
Instructions for use
Dropper (for test cassette only)
Desiccant
[MATERIALS REQUIRED BUT NOT PROVIDED]
Disposable specimen container
Wimer
[PRECAUTIONS]
· Please read all the information in this instruction for use before performing the test.
· Do not use after the expiration date printed on the foil pouch.
· Store in a dry place at 4-30*C. Do not freeze.
· Do not use if pouch is torn or damaged.
· Keep out of the reach of children.
· For in vitro diagnostic use. Not to be taken internally.
· Do not open the test foil pouch until you are ready to start the test.
· The used test should be discarded according to local regulations.
· Test is for single use only. Do not re-use under any circumstances.
· The used testing materials may contain sources of infection or other biohazards, so please handle them with caution.
· Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the Member State.
· For near-patient testing, the user shall be normal licensed physician or nurse.
[STORAGE AND STABILITY]
· Store in the sealed pouch at 4'C to 30°C. The validity period is 36 months.
· The test is stable through the expiration date printed on the sealed pouch.
· The test must remain in the sealed pouch until use.
· After opening the sealed pouch, use the test as soon as possible within 60 minutes.
· Do not freeze.
· Do not use beyond the expiration date.
[SPECIMEN COLLECTION AND STORAGE]
· Fresh urine specimen must be collected in a clean and dry container, The first-morning urine is the best.
· Limit liquid drink intake 2 hours before urine specimen collection.
· Urine specimens exhibiting visible particles should be centrifuged, filtered, or allowed to settle to obtain clear specimen for testing.
· Perform the testing immediately after the specimen collection, lf the urine samples cannot be tested in time, the urine samples can be stored at room temperature for 4 hours or stored at 2-8'C for up to 48 hours.
· If specimen has been refrigerated, leave it at room temperature for about 30 minutes before testing.
[PROCEDURE]
Preparing for a test
· Equilibrate the tests, urine specimens to room temperature(15-25°C) prior to testing.
· Carefully read the instructions for use before performing the test.
· Urinate into a clean, dry cup or container.
· Remove the test from the sealed pouch and use it as soon as possible
For Test Strip:
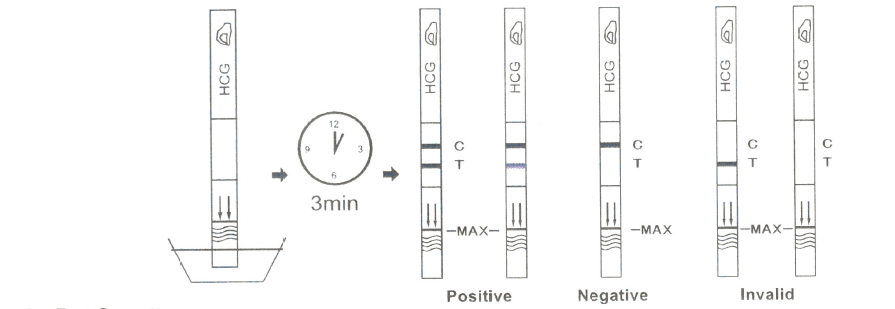
· With arrows pointing toward the urine, dip the test strip vertically in the urine for at least 10-15 seconds. Do not pass the maximum line (MAX) on the test strip when dipping the strip into the urine.
· Remove the test strip from the urine, place it on a flat dry surface.
· Start the timer and wait for the colored line(s) to appear.
For Test Cassette :
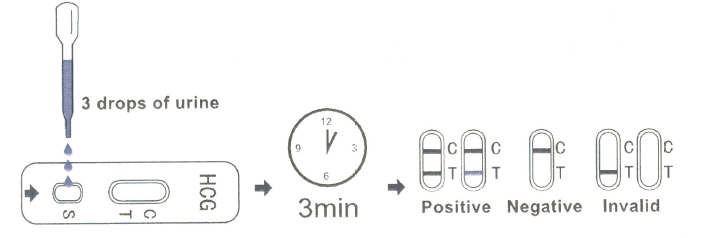
· Place the test cassette on a clean and level surface. Hold the dropper vertically and transfer 3 full drops of urine to the specimen well(s) of the test cassette. Avoid trapping air bubbles in the specimen well(s).
· Start the timer and wait for the colored line(s) to appear.
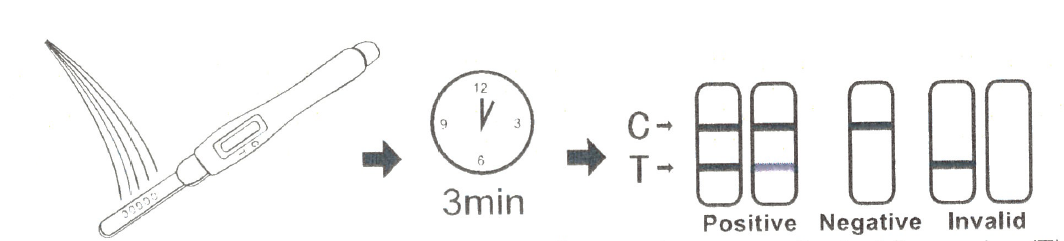
For Test midstream:
· Remove the cap from the end of the test midstream.
· Pour the urine directly on the absorbent tip of the midstream, and make sure that the urine do not exceed the arrow on the midstream. Make the urine as wet as possible on the absorbent tip, put it flat when urine climbs to the observation window.
· If you prefer, you can urinate into a clean and dry container, then dip only the absorbent tip of the midstream test into the urine for at least 10-15 seconds, Do not pass the arrow on the midstream when immersing the midstream, Remove the test midstream from the urine, Place it on a flat dry surface.
· Start the timer and wait for the colored line(s) to appear.
[INTERPRETATION OF RESULTS]

[LIMITATIONS]
1. The human chorionic gonadoptropin (HCG) pregnancy test kit is a preliminary qualitative test, therefore, neither the quantitative value nor the rate of increase in HCG can be determined by this test.
2. Very low levels of hCG (less than 50 mlU/mL) are present in urine specimens shortly after implantation. However, because a significant number of first trimester pregnancies terminate for natural reasons, a test result that is weakly positive should be confirmed by retesting with a first morning urine specimen collected 48 hours later.
3. This test reliably detects intact HCG up to 200.000mlU/mL. It does not reliably detect hCG degradation products including free-beta hCG and beta core fragments. Quantitative assays used to detect hCG may detect hCG degradation products and therefore may disagree with the results of this rapid test.
4. This test may produce false positive results, A, number of conditions other than pregnancy, including trophoblastic disease and certain non-trophoblastic neoplasms including testicular tumors, prostate cancer, breast cancer, and lung cancer cause elevated levels of hCG. Therefore, the presence of hCG in urine should not be used to diagnose pregnancy unless these conditions have been ruled out.
5. This test may produce false negative results. False negative results may occur when the levels of hCG are below the sensitivity level of the test. When pregnancy is still suspected, a first morning urine specimen should be collected 48 hours later and tested. In case pregnancy is suspected and the test continues to produce negative results, see a physician for further diagnosis.
6. This test provides a presumptive diagnosis for pregnancy. A confirmed pregnancy diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
[PERFORMANCE CHARACTERISTICS]
1. Accuracy
The comparison studies were conducted using Human Chorionic Gonadotropin (HCG) Pregnancy Test Kit (Colloidal Gold and commercially available tests, The study included 450 urine specimens, and both assays identified 225 negative and 225 positive results. The results were >99% in agreement.
2. limit of Detection
The Limit of Detection was determined by tested controls which has been standardized to the WHO International Standard.
The Limit of Detection is 25mlu/mL. This test kit detects HCG at a concentration of 25mlW/mL or greater.
3. Reproducibility
The results of HCG standard at the same concentration were consistent and the chromaticity was uniform.
4. Analytical Specificity
· The addition of LH (500mlU/mL), FSH (1.000mlU/mL) and TSH (1,000μlU/ml) to negative and positive specimens showed no cross-reactivity.
· Interfering Substances
The following potentially interfering substances were added to HCG negative and positive specimens, which did not interfere the test result.
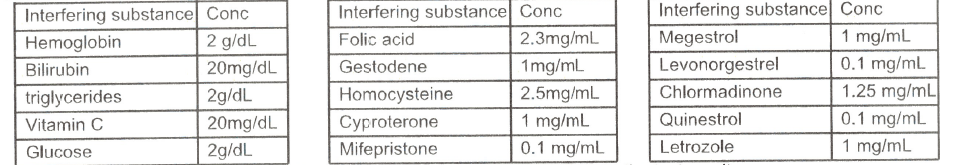
Different urine pH (3-10) and specific gravity (1.002-1.030) has no effect on the test result.
5. HOOK effect
The concentration of HCG was 200lU/mL, there was no obvious HOOK effect.
[QUESTIONS & ANSWERS]
1. Q: How does the test work?
A: This test kit detects a hormone in your urine that your body produces during pregnancy (HCG-human chorionic gonadotropin). The amount of pregnancy hormone increases as pregnancy progresses.
2. Q: How soon after l suspect that I am pregnant can I take the test?
A: You can test your urine as early as 7 days before your next period. You can perform the test any time of the day; however, if you are pregnant, first morning urine contains the most pregnancy hormone.
3. Q: Do l have to test with first morning urine?
A: Although you can test at any time of the day, your first morning urine is usually the most concentrated of the day and would have the most hCG in it.
4. Q: How do l know that the test was run properly?
A: The appearance of a colored line in the control region (C) tells you that you followed the test procedure properly and the proper amount of urine was absorbed.
5. Q: What should l do if the result shows that l am pregnant?
A: it means that your urine contains HCG and you are probably pregnant. See your doctor to confirm that you are pregnant and to discuss the steps you should take.
6. Q: What should l do if the result shows that l am not pregnant?
A: lt means that no hCG has been detected in your urine and probably you are not pregnant. If you do not start your period within a week of its due date, repeat the test with a new test strip, if you receive the same result after repeating the test and you still do not get your period, you should see your doctor. You are encouraged to take the following steps to increase your chances for a healthy pregnancy and a healthy baby.
· Use the HCG pregnancy test kit to detect pregnancy when your period is late. You can begin better prenatal care as soon as you learn of your pregnancy.
· If you get a positive result, it is advisable to visit your doctor immediately to begin your prenatal care.
· Maintain a well-balanced diet, stop smoking, and reduce your intake of alcohol
Latest version 8.29.04.0105 A3 lssued Date: 2024.05.08