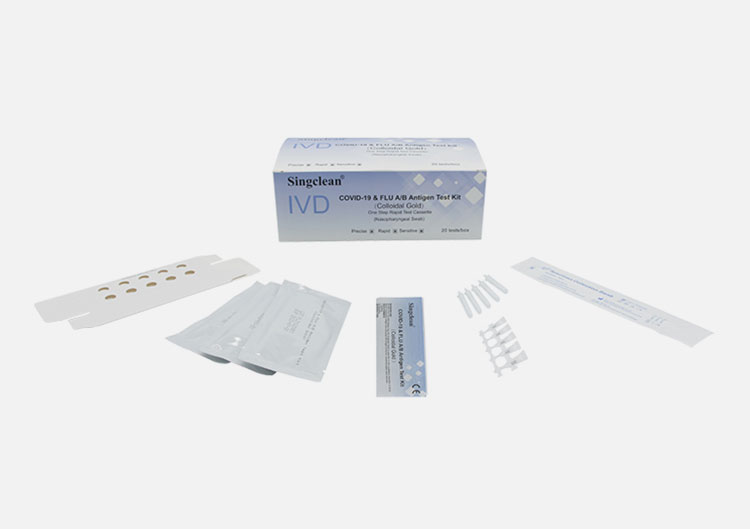
CE COVID-19 & FLU A/B Antigen Test Kit (Colloidal Gold)
CE
INTENDED USE
COVID-19 & FLU A/B Antigen Test Kit (Colloidal Gold) is used for in vitro qualitative detection of 2019 Novel Coronavirus antigen and influenza A/B antigen in human nasopharyngeal swab samples.
PACK FORMATS
1 Test/Box
20 Tests/Box
INTRODUCTION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease.People are generally susceptible.Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatically infected people can also be an infectious source.Based on the current epidemiologicai investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days.The main manifestations include fever, fatigue and dry cough.Nasal congestion, runny nose, sore throat,myalgiaand diarrhea are found in a few cases.
Influenza A/B viruses(Flu A/B) can cause acute respiratory infections,and people are generally susceptible.These two viruses are highly contagious, spread quickly, have a short incubation period, and have a high incidence.The main symptoms are fever, dry cough, fatigue, etc. Therefore, the detection of COVID-19, Flu A/B has relatively great clinical findings.
PRINCIPLE
These two tests use colloidal gold immunochromatography to detect COVID-19 and Flu A/B antigens. The test of COVID-19 use COVID-19 monoclonal antibody (test line T) and goat anti-mouse polyclonal antibody (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to another COVID-19 monoclonal antibody. When the processed buffer containing the sample is added to the sample well, COVID-19 will combine with the COVID-19 monoclonal antibody conjugate to form an antigen-antibody complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the COVID-19 monoclonal antibody of test line T, the complex is trapped forming a burgundy colored band which confirms are active test result: Absence of a colored band in the test means negative test result.
The test of Flu A/B is coated with influenza A virus monoclonal antibody (test line A), influenza B virus monoclonal antibody (test line B) and goat anti-mouse polyclonal antibody (control line C) on a nitrocellulose membrane. The gold label pad contains another influenza A virus monoclonal antibody and influenza B virus monoclonal antibody coupled with colloidal gold. When a sample containing influenza A virus antigen is added to the sample well, the influenza A virus antigen will combine with the influenza A virus monoclonal antibody conjugate to form an antigen-antibody complex. The complex moves through the nitrocellulose membrane. When the complex encounters the influenza A virus monoclonal antibody on the test line A, the complex is captured and forms a burgundy band, which confirms the positive test result. When a sample containing influenza B virus antigen is added to the sample well, the influenza B virus antigen is the same as that of influenza A virus antigen. The influenza B virus antigen will color the test line B, forming a burgundy line, that is, confirm Influenza B virus positive test result. No color band on test line A/B means negative fest result.
These two tests contain a quality control line (control line C),which should show a purple-red color band of goat anti-mouse polyclonal antibody combined with the colloidal gold conjugate in the gold label pad, regardless of the color on any other test line.
MATERIALS SUPPLIED
1.Sampling cotton swabs (as nasopharyngeal swab)
2.Antigen extraction buffer
3.Antigen extraction tube
4.Paper workbench(The small one-test-box can be used as a workbench)
5.lnstruction for use
MATERIAL REQUIRED BUT NOT PROVIDED
Timer
STORAGE AND STABILITY
The test kit can be stored at room temperature or refrigerated (4-30°C).The test device is stable through the expiration date printed on the sealed pouch.The test device must remain in the sealed pouch until use.
Do not freeze.
Do not use beyond the expiration date.
After opening the sealed pouch, use the test as soon as possible within 60 minutes.
WARNINGS AND PRECAUTIONS
1. For professional In vitro diagnostic use only. Do not use after expiration date.
2. These instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All users have to read the instruction prior to performing a test.
3. Do not use it if the tube/pouch is damaged or broken.
4. Wear protective gloves while handling specimens and wash hands thoroughly afterwards.
5. Avoid splashing or aerosol formation of specimen and buffer.
6. Clean up spills thoroughly using an appropriate disinfectant.
7. Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials (i.e. swab, extraction tube, test device) in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
8. Do not mix or interchange different specimens.
9. Do not mix reagent of different lots or those for other products.
10. Do not store the test kit in direct sunlight.
11. To avoid contamination, do not touch the head of provided swab when opening the swab pouch.
12. The provided swabs in the package should be used only for nasopharyngeal specimen collection.
13. To avoid cross-contamination, do not reuse the swabs for specimen collection.
14. Do not dilute the collected swab with any solution except for the provided extraction buffer.
15. Test is for single use only. Do not re-use under any circumstances.
16. Do not perform the test in a room with strong air flow, ie. electric fan or strong air-conditioning.
SPECIMEN COLLECTION
1. COVID-19 & FLU A/B Antigen Test kit (Colloidal Gold)can be performed using nasopharyngeal swab.
2. Testing should be performed immediately after specimen collection.
3. Bring specimens to room temperature prior to testing.
4. If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
TEST PROCEDURE
Allow test cassette, antigen extraction buffer, specimen and or controls to equilibrate to room temperature(15-30°C) prior to testing.
1.Ask the patient to remove the secretions on the surface of the anterior nasal cavity, keep the heads lightly tilted, and gently and slowly insert the swab through the nasal cavity to the nasopharynx, When resistance is encountered, it will reach the posterior nasopharynx, stay for a few seconds to absorb secretions, and gently rotate to remove the swab.
2.Place the antigen extraction tube on the workbench. Place the antigen extraction buffer bottle vertically downward, squeeze the bottle to make the buffer drip freely into the antigen extraction tube without touching the edge of the tube, and add 8 drops (about 300ul) to the antigen extraction tube.
3.Put the swab specimen into the antigen extraction tube pre-added with the antigen extraction buffer, and rotate the swab about 10 times while pressing the swab head against the tube wall to release the antigen in the swab, then let it stand for about 1 minute.
4.Remove the swab while squeezing the tip of the swab so that as much liquid in the swab can be discharged as possible. Dispose of used swabs in accordance with biohazard waste disposal methods.
5.Instail the dripper on the antigen extraction tube and cap it tightly, and let it stand for about 1 minute.
6.Remove the test cassette from the sealed foil pouch and use it as soon as possible
7.Place the test device on a clean and level surface.
8.Transfer 3 drops(about 100 ul) of mix liquids to the sample well of the test card (or use a pipette to add 100 ul),and start the timer.
9.Wait for the test result of the reagent. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
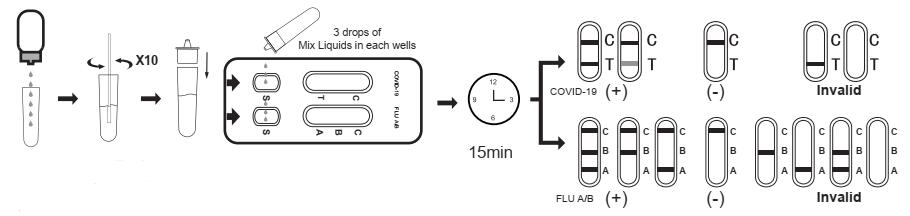
INTERPRETATION OF RESULTS
For COVID-19 Antigen test kit:
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no COVID-19 antigen is detected in the specimen. The result is negative.
POSITIVE:
In addition to the presence of C band, if T band is developed, the test indicates for the presence of COVID-19 antigen in the specimen. The result is COVID-19 positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette.
If the problem persists, discontinue using the test kit immediately and contact your local distributor.
For Flu A/B Antigen test kit:
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no Flu A/B antigen is detected in the specimen. The result is negative.
POSITIVE
FLU A/B positive:
In addition to the presence of the C-line, If the test lines A and B appears at the same time, it means that there are both influenza A virus antigen and influenza B virus antigen in the sample, that is, the result is positive for FLU A and FLU B.
FLU A positive:
In addition to the presence of the C-line, if the test line A appears, the test indicates the presence of FLU A antigen in the sample, that is, the result is positive for FLU A.
FLU B positive:
In addition to the presence of the C-line, if the test line B appears, the test indicates the presence of FLU B antigen in the sample, that is, the result is positive for FLU B.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette.
If the problem persists, discontinue using the test kit immediately and contact your local distributor.
LIMITATIONS
1.Use fresh samples whenever possible.
2.Optimal assay performance requires strictly adherence to the assay procedure described in Instruction for use. Deviations may lead to aberrant results.
3.A negative result for an individual subject indicates absence of detectable COVID-19 or Flu A/B antigen. However, a negative test result does not preclude the possibility of exposure to or infection with COVID-19 and Flu A/B.
4.A negative result can occur if the quantity of the COVID-19 or Flu A/B antigen present in the specimen is below the detection limits of the assay, or failed to collect the COVID-19 or Flu A/B antigen in the nasal cavity of the patient.
5.As with all diagnostic tests, a definitive clinical diagnosis should not be based on the reslt of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
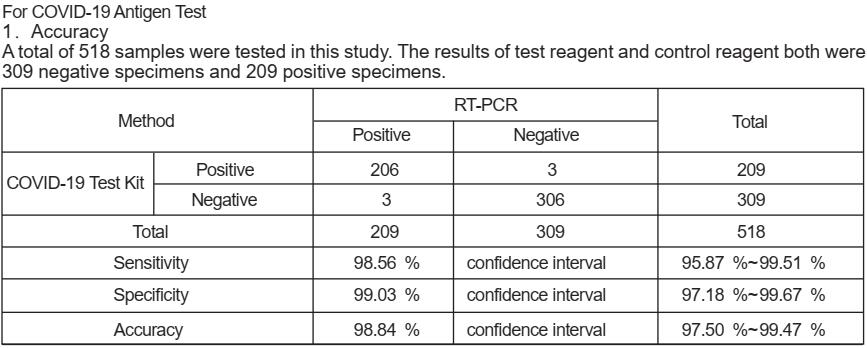
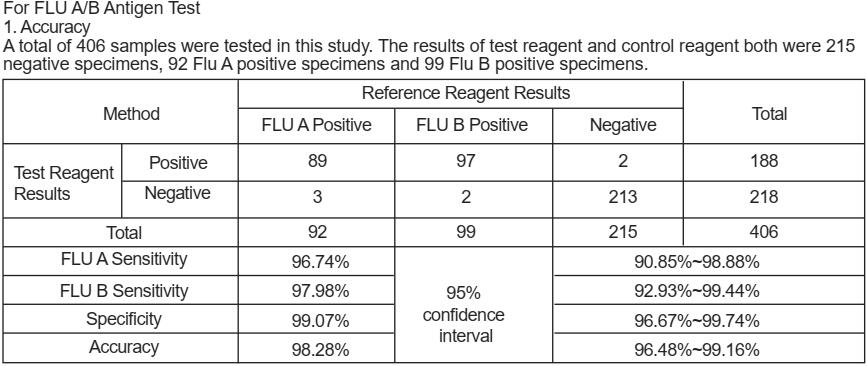
PERFORMANCE CHARACTERISTICS
Latest version: 8.53.04.001-A5 Issued Date: 2022-12-30