CE Singclean® COVID-19 IgG/IgM Test kit(Colloidal Gold Method)
CE
INTENDED USE
COVID-19 IgG/IgM Test kit(Colloidal Gold Method)is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM antibodies to Novel Coronavirusi in human whole blood, serum or plasma. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Test kit(Colloidal Gold Method) must be confirmed with alternative testing method(s) and clinical findings.
PACK FORMATS
1 test/box ,20 test/box,50 test/box ,100 test/box
INTRODUCTION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The COVID-19 IgG/IgM Test kit(Colloidal Gold Method)uses the principle of colloidal gold immunochromatography. The test uses anti-human IgM antibody (test line IgM), anti-human IgG (test line IgG) and goat anti-mouse IgG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to recombinant COVID-19 antigens conjugated with colloid gold (COVID-19 conjugates). When a specimen followed by assay buffer is added to the sample well, IgM &/or IgG antibodies if present, will bind to COVID-19 conjugates making antigen antibodies complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the corresponding immobilized antibody (anti-human IgM &/or anit-human IgG) the
complex is trapped forming a burgundy colored band which confirm a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
Regardless of the presence of COVID-19 IgG/IgM antibody in the test sample, a colored band will appear on the quality control line (line C). The colored band appearing on the quality control line (line C) is used to determine whether there are enough samples and whether the chromatographic process meets the normal standard, and also serves as the internal control standard of the reagent.
MATERIALS SUPPLIED
Sealed pouches each containing a test cassette, a desiccant dropper
Lancets(for fingerstick whole blood only)
sterilizing tablet(for fingerstick whole blood only)
Buffer
Package insert
MATERIAL REQUIRED BUT NOT PROVIDED
Specimen collection containers 2. Centrifuge (for plasma only) 3. Timer
STORAGE AND STABILITY
The kit can be stored at room temperature or refrigerated (4-30°C). The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.
DO NOT FREEZE. Do not use beyond the expiration date.
WARNINGS AND PRECAUTIONS
1. For professional In Vitro diagnostic use only. Do not use after expiration date.
2. This package insert must be read completely before performing the test. Failure to follow the insert gives inaccurate test results.
3. Do not use it if the tube/pouch is damaged or broken.
4. Test is for single use only. Do not re-use under any circumstances.
5. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens.
6. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
7. Humidity and temperature can adversely affect results.
8. Do not perform the test in a room with strong air flow, ie. electric fan or strong air-conditioning.
9. COVID-19 IgG/IgM Test Kit (Colloidal Gold Method) (hereinafter referred as Product) used to detect antibodies produced by human body to against COVID-19 after infected.COVID-19 positive patients may be tested negative by this Product at that period, as human body has not produced or produced very few antibodies at that period, which can’t be detected by the Product.
10. Product can be only used as auxiliary diagnosis for patients infected with COVID-19 and should not be the final diagnosis of COVID-19 infection. It must be combined with other medical tests for comprehensive diagnosis.
11. The Product is designed for use by healthcare professionals. It should be operated by healthcare professionals. Individuals are not allowed to use.
12. The used reagent and accessories should also be treated as the source of infection and treated as biohazard wastes.
SPECIMEN COLLECTION
1. COVID-19 IgG/IgM Test kit(Colloidal Gold Method) can be performed using either whole blood, serum or plasma.
2. Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear, non-hemolyzed specimens.
3. Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8°C for up to 3 days. For long term storage, specimens should be kept below -20°C. Whole blood collected by venipuncture should be stored at 2-8°C if the test is to be run within 2 days of collection. Do not freeze whole blood specimens. Whole blood collected by fingerstick should be tested immediately.
4. Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
5. If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
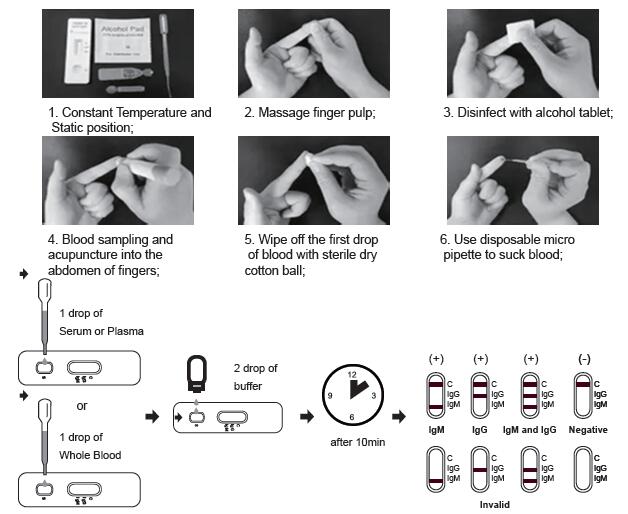
TEST PROCEDURE
Allow test cassette, specimen, buffer and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Remove the test cassette from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
2. Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
With the provided dropper, draw serum/plasma specimen and then add 1 drop serum/plasma specimen into the sample well(S). Then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
For Whole Blood Specimen:
With the provided dropper and transfer 1 drop of whole blood to the sample well(S) of the test device, then add 2 drops of buffer to the sample well(S) immediately. Avoid air bubbles.
3. Wait for the colored line(s) to appear. The result should be read after 10 minutes. Do not interpret the
result after 20 minutes.
OPERATION INSTRUCTIONS
correct use, convernient and quick
INTERPRETATION OF RESULTS
NEGATIVE: If only the C band is present, the absence of any burgundy color in the both T bands (IgG and IgM) indicates that no anti-COVID-19 antibodies are detected in the specimen. The result is negative.
IgM POSITIVE:
In addition to the presence of C band, if only IgM band is developed, the test indicates for the presence of IgM anti-COVID-19 in the specimen. The result is IgM anti-COVID-19 positive.
IgG POSITIVE:
In addition to the presence of C band, if only IgG band is developed, the test indicates for the presence of IgG anti-COVID-19 in the specimen. The result is IgG anti-COVID-19 positive.
IgG and IgM POSITIVE:
In addition to the presence of C band, both IgG and IgM bands are developed, the test indicates for the presence of both IgG and IgM anti-COVID-19 in the specimen. The result is IgG and IgM anti-COVID-19 positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
QUALITY CONTROL
A procedural control is included in the test. A red line appearing in the control region (C) is the internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Control standards are not supplied with this kit; however it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
LIMITATIONS
1. Use fresh samples whenever possible. Frozen and thawed samples (especially repeatedly) contain particles that can block the membrane. This slows the flow of reagents and can lead to high background color, making the interpretation of results difficult.
2. Optimal assay performance requires strict adherence to the assay procedure described in this insert sheet. Deviations may lead to aberrant results.
3. A negative result for an individual subject indicates absence of detectable anti-COVID-19 antibodies. However, a negative test result does not preclude the possibility of exposure to or infection with COVID-19.
4. A negative result can occur if the quantity of the anti-COVID-19 antibodies present in the specimen is below the detection limits of the assay, or the antibodies that are detected are not present during the stage of disease in which a sample is collected.
5. Some specimens containing unusually high titer of heterophile antibodies or rheumatoid factor may affect expected results.
6. As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
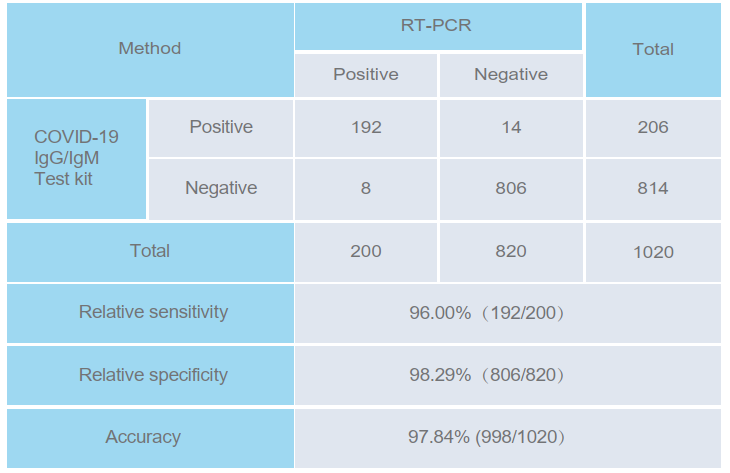
PERFORMANCE CHARACTERISTICS
The COVID-19 IgG/IgM detection kit (whole blood/serum/plasma) was evaluated with 1020 specimens. The
results were compared with clinical diagnosis (based on RT-PCR).
REFERRENCE
1. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011; 81: 85-164.
2. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses.
Trends Microbiol 2016; 24: 490-502.
Latest version 8.127.04.020 A6 Issued Date: 2022-12-30