CE Singclean Monkeypox Virus Nucleic Acid Detection Kit
(Fluorescence PCR)
CE
[Product Name]
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR)
[Packaging Specification]
25 test/box, 50 tests/box
[Intended Use]
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) is used for the detection of nucleic acid from monkeypox virus in serum and human pustular or vesicular rash specimens from individuals suspected of monkeypox virus infectious. By specifically detecting the nucleic acid fragments of monkeypox virus, monkeypox virus can be quickly identified, which is suitable for rapid diagnosis of related diseases caused by monkeypox virus infection.
[Test Principle]
This kit uses polymerase chain reactor-fluorescent probe technology to design specific primers according to the nucleic acid sequence of monkeypox virus for amplification of corresponding nucleic acid fragments. At the same time, the highly specific probe can be combined with the corresponding nucleic acid fragment and hydrolyzed under the action of exonuclease activity to generate fluorescence signal for detection. Real-time amplification curve can be obtained according to the relationship between fluorescence signal and amplification cycle number.
[Kit Component]
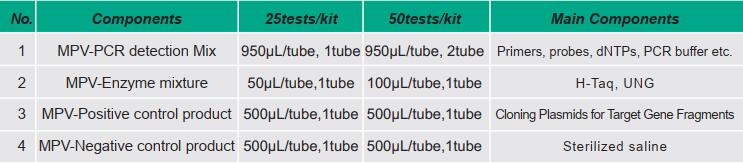
Note:
1.Kits with different batch numbers are not interchangeable.
2.All biological samples in the kit have been extinguished.
[Materials Required but Not Provided]
1) 48 or 96-well Polypropylene PCR Microplate. Or 0.2 mL Polypropylene PCR Tube Strips with Flat Cap,
2) Nucleic acid extraction reagents or Preservation solution,
3) Pipettes and Pipetting tips (10μL, 200μL and 1000μL tips with filters),
4) Centrifuge,
5) Desktop vortex mixer,
6) Disposable powder-free gloves and surgical gowns
[Applicable Instrument]
Recommended PCR instruments include: Applied Biosystems™ 7500 real-time PCR system; ABI 7500 Real-time fluorescence quantitative PCR instrument , SLAN®-96S/96P real-time PCR system, Thermofisher QuantStudio™5 Real-time fluorescence quantitative PCR, MA-6000 Real-Time Quantitative Thermal Cycler.
[Sample Requirement]
1.Sample type
Serum and human pustular or vesicular rash specimens.
2.Sample collection
Serum: Use a sterile syringe to draw 2ml of the subjects venous blood, inject it into a sterile collection tube, leave it at room temperature for no more than 4 hours, wait for the sample to separate out serum, or centrifuge at 4000 rpm for 5 minutes at room temperature to separate the serum, and transfer it to a 1.5ml sterile spare centrifuge tube Sample collection of vesicles and pustules: Select fresh vesicles or pustules on the skin, disinfect them with iodophor, and then wipe the iodophor with 70% alcohol. After the alcohol is completely evaporated, use a sterile needle to puncture and squeeze out the tissue fluid, dip the cotton swab into the liquid, quickly put the cotton swab into the 3-5 mL sampling tube containing the preservation solution, break the cotton swab near the top, tighten the tube cap, and seal it for inspection.
3. Sample Storage and Shipping
The collected specimens should be sent for testing immediately. Specimens should be tested within 24 hours if stored at 2℃ to 8℃. Specimens that cannot be tested within 24 hours should be stored at -70 C or below (in the absence of-70℃ storage conditions, specimens can be stored at -25℃ to -15℃ for 10 days). Multiple freeze/thaw cycles should be avoided. Specimens should be transported in a sealed frozen pitcher with ice or in a sealed foam box with ice.
[Test Method]
Please read the instructions of the test kit before use.
1.Reagent preparation (in the reagent preparation area)
1.1 Take out the components in the box and place them at room temperature. After the temperature is equilibrated to room temperature, mix well and centrifuge briefly for later use. According to the number of samples to be tested, negative controls, and positive controls, take the corresponding amount of components in proportion (38μL/person of MPV-PCR reaction solution + 2μL/person of MPV-enzyme mixture), and mix them thoroughly to form a PCR mixture. Liquid-1, which was used after instant centrifugation.
1.2 Transfer the above-prepared reagents to the sample processing area for use.
2.Sample processing (in the sample processing area)
2.1 Sample extraction:
Nucleic acid extraction kit should be used for sample extraction.
Negative control, positive control and samples to be tested are processed simultaneously.
2.2 Add sample
Add 10μL of extracted DNA to PCR tubes in the following order: MPV negative control, MPV positive control, and sample to be tested. Add 40μL of MPV master mix to each well. Each well was capped. centrifuged at 2000 rpm for 10 seconds, and placed on a fluorescent PCR machine.
3.PCR amplification (in the amplification and analysis area)
Please refer to the instruction manual of each instrument for settings.
3.1 Put the PCR reaction tubes that have finished adding samples into the fluorescence PCR instrument, set test holes according to the corresponding sequence of adding samples, set test holes for positive quality control products, negative quality control products and unknown samples, and set the sample name.
3.2 Total reaction system: 25μL
3.3 Fluorescence detection channel selection:1) Select the FAM channel (Reporter: FAM, Quencher: none) to detect monkeypox virus target genes: 2) Select the HEX or VIC channel (Reporter: HEX/VIC, Quencher: none); 3)Set the reference fluorescence (Passive Reference) to none ; Set Sample Volume to 50.
3.4 Cyclic parameter setting:
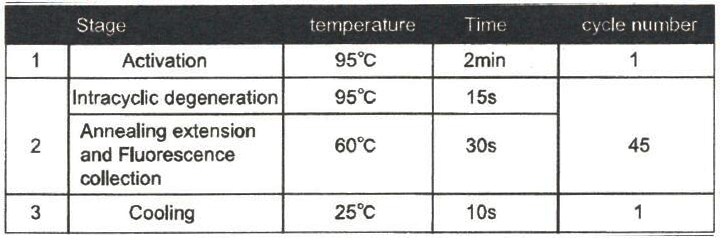
Note: Use ABI 7500 Rea-time fluorescence quantitative PCR instrument, and please choose "None" at passive reference and quencher.
4.Result analysis
After the reaction, the results will be automatically saved, and the Baseline and Threshold of each detection channel will be adjusted according to the analyzed images. Click "Analysis" for analysis to make all parameters meet the requirements of quality control, and then check the detection results of each unknown sample.
5.Quality control
Negative control products: FAM curve has no Ct value or Ct>40; and monkeypox virus internal standard detection (HEX/VIC) has no Ct value or Ct>40.
Positive control products: FAM curve CS35; and monkeypox virus internal standard detection (HEX/VIC) is positive, Ct≤35.
lf the above two items are met at the same time in the same inspection, otherwise, the second inspection shall be deemed invalid and re-inspection shall be required.
[Positive reference value]
Through the study of reference values, it was determined that the reference value of Ct of the target gene detected by this kit was 40, and the reference value of Ct of the internal standard was 40.
[Interpretation of test results]
1.FAM detection Ct value ≤ 40, and the internal standard detection is positive (Ct value ≤ 40), reported as monkeypox virus positive;
2.No Ct value or Ct value > 40 in FAM detection, and the internal standard channel detection is positive (Ct value ≤ 40), and the report is negative for monkeypox virus;
3.lf there is no Ct value or Ct value > 40 in the internal standard test, the test result of the sample is invalid. The cause should be found and eliminated, and the sample should be re-sampled and tested again (if the test result of the repeated test is still invalid, please contact the company).
4.For positive samples, the internal standard test result is not required; for negative samples, the internal standard test should be positive. lf the internal standard test is negative, the test result of the sample is invalid. Samples are re-sampled and repeated experiments are carried out (if the test results of repeated experiments are still invalid, please contact our company).
[Limitations of the test method]
a)The test results need to be combined with other clinical and laboratory data, and if the test results do not match the clinical assessment, further testing is required.
b)Recent or remote vaccination with a vaccinia-based vaccine (e.g.anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive results.
c)Other factors, including technical reasons, operational errors and other sample factors, may also cause errors in test results.
[Performance Index]
1. Specificity: The kit has no cross-reaction with measles virus, rubella virus, parvovirus B19, varicella zoster virus, syphilis virus and herpes simplex virus.
2. Minimum detection limit: 400 copies/mL.
3. Precision: The coefficient of variation (CV %) of the Ct value of the intra-assay precision is less than or equal to 5%.
4. Interfering substances that may exist in the sample: hemoglobin (≤ 2mg/dL), total bilirubin (≤ 28mg/dL), triglyceride (≤ 3g/dL), total IgG (≤ 40mg/mL) have no effect on the detection. Influence; paracetamol (≤ 60μg/mL) has no influence on the detection.
[Precautions]
1.This kit is for professional use only.
2.Standard precautions should be followed. All patient samples and positive controls should be considered potentially infectious and handled accordingly.
3.Handle hazardous or biocontaminated materials in accordance with your institution's practices.
4.Please read the instructions carefully before operation. This kit is only for emergency use in in vitro diagnostic testing. During the experiment, from specimen collection and storage to laboratory testing, each step of the operation should be strictly in accordance with relevant biosafety regulations and molecular experiments.
5.A separate laboratory area is required for the execution of predetermined procedures. a) Area 1: preparation area-preparing detection reagents b)Area 2: sample processing area-processing samples and controls c) Area 3:amplification area-PCR reaction area.
6.All test samples must be treated as infectious substances. Change lab coats and disposable gloves to avoid cross-contamination between samples. Disposal of samples and waste must comply with local, state and national regulations.
Latest version 8.73.04.006 A1 lssued Date: 2023.01.04