CE 2460
Hyaclean®️ (Medical sodium hyaluronate gel) is clear liquid gel, which is compounded sodium hyaluronate and physiological balancing salt. Hyaluronic Acid (herein after ‘HA’)is a unique type of linear macromolecular mucopolysaccharide, composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine, in chemical formula of (C14 H21No11)n. Hyaclean®️ is a high molecular weight (between 1.000,000-2.600,000) fraction of sodium hyaluronate (1.5% total weight) dissolved in a sodium phosphate buffer. The osmotic pressure of Hyaclean is nominally 270 mOsmol/kg~350mOsmol/kg.
Component:
Hyaclean is processed with moist heat terminal sterilization. The product is for single use only. its final packagingincludes one 27G blunt needle which is terminal sterilized by EO.

Indications :
Hyaclean is intended to aid anterior or posterior segment ophthalmic surgery, including:
Cataract extraction with/without implantation of intraocular lens;
Corneal transplant surgery;
Glaucoma filtering surgery:
Secondary lens implantation.
Contraindications:
Patients with allergy background should not use this product.
Precautions:
Overfilling the eye anterior or posterior segment with Hyaclean may lead to increased intraocular pressure, glaucoma or other ocular damage.
Postoperative intraocular pressure may also be elevated as a result of pre-existing glaucoma, compromised outflow and by operative procedure and seguel a thereto, including enzymatic zonulysis, absence of an indectomy, trauma to filtration structures, and by blood and lenticular remnants in the anterior chamber.
It is dificult to predict the exact role of these factors in any individual case, therefore the following precautions are recommended:
Never overfill the eye chambers with Hyaclean® (except in glaucoma surgery).
In posterior segment procedures in the aphakic diabetic patients, special care should be exercised to avoid using large amounts of Hyaclean®.
Remove all remain Hyaclean by irrigation and/or aspiration at the close of surgery (except in glaucoma surgery).
Carefully monitor the intraocular pressure especially during the immediate postoperative period. lf significant rises are observed, treat with appropriate therapy.
Hyaclean is obtained from microbial fermentation by a highly purified proprietary process. The physician should be aware of potential allergic risks that can occur with the injection of any biological material.
Needle is for single use only.
Avoid trapping air bubble.
On rare occasion, viscoelastic Hyaclean containing sodium hyaluronate has been observed to become slightly opaque or to form a slight precipitated upon instillation into the eye. Should this be observed, the cloudy or precipitated material should be removed by irrigation and/or aspiration.
Use only if solution is clear.
The graduation scale on the syringe label is only indicative
Adverse reactions:
Hyaclean is extremely well tolerated after injection into human eyes. A transient rise of intraocular pressure postoperatively has been reported in some cases. In posterior segment surgery intraocular pressure rise have been reported in some patients, especially in aphakic diabetics, after injection of large amounts of Hyaclean®.
Rarely, postoperative inflammatory (iritis, hypopyon) as well as incidents of corneal edema and corneal decompensation have been reported. Their relationship to Hyaclean has not been established.
Application:
Cataract surgery and lOL implantation
The required amount of Hyaclean is slowly infused through a needle or cannula into the anterior chamber. The protective effect of Hyaclean as an aid is optimized when the injection is performed prior to cataract extraction and insertion of the lOL and is effective for both intra- and extra-capsular procedures. Hyaclean may be applied to lOL prior to insertion. Additional Hyaclean can be injected as required to facilitate surgical procedures.
Corneal transplant surgery
The corneal button is removed and the anterior chamber filled with Hyaclean is at level with the surface of the corneal. The donor graft is then placed on top of the Hyaclean and sutured into place. Additional Hyaclean can be used as required to aid in surgical procedures.
Glaucoma filtration surgery
Hvaclean is injected through a corneal paracentesis to restore and maintain anterior chamber volume during the performance of the trabeculectomy. Additional Hyaclean can be used as required to aid in the surgical procedures.
Installation Notes:
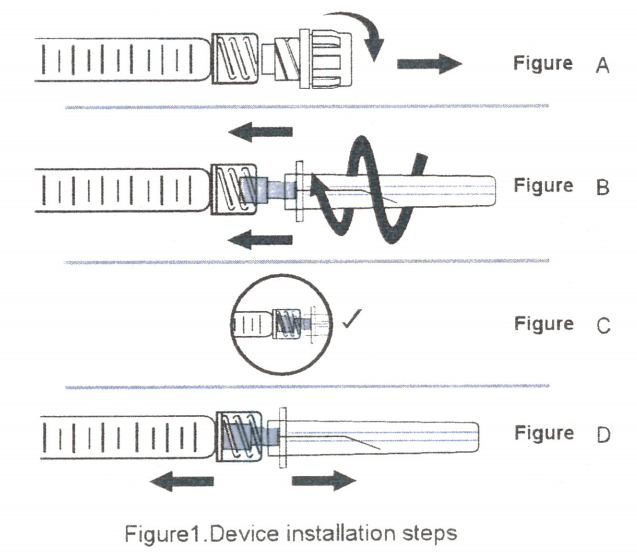
The first step is to remove the tip cap: take the prefilled syringe out of the blister, hold the back end of prefilled syringe, and unscrew the tip cap from the syringe, as shown in Figure A.
The second step is to insert the blunt needle: hold the back of the prefilled syringe and insert the blunt needle into the luer end of the syringe exactly, as shown in Figure B.
The third step is to tighten the blunt needle: rotate and tighten the needle clockwise (as shown in Figure B) to the proper position, as shown in Figure C.
The fourth step is to remove the needle cap: hold the syringe and needle cap with both hands, and pull the needle cap in the opposite direction (do not rotate), as shown in Figure D.
Direction for use:
First expulse the air out of the ophthalmic Luer-lock needle, then inject Hyaclean® appropriately into eye. The injected amount must be adapted to the type of surgical procedures. The administration of Hyaclean® should be performed only by trained specialists.
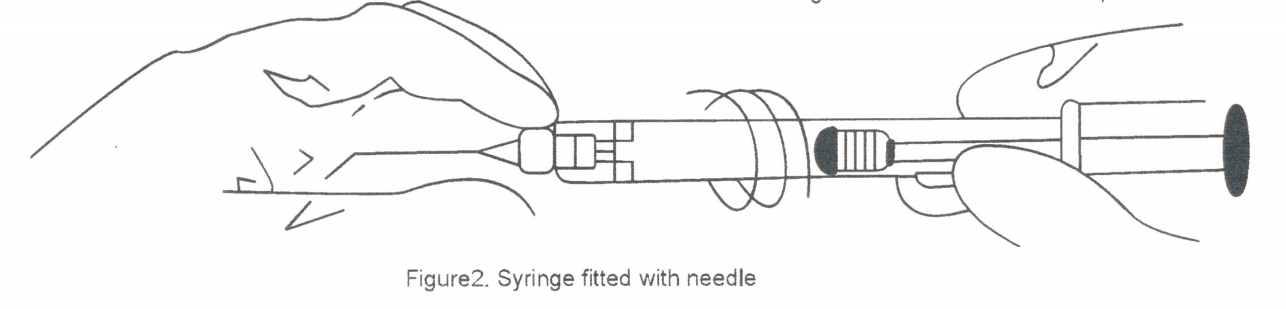
The needle should fit to the syringe luer tip (shown in figure 2); however, over tightening may cause the hub to weaken and possibly detach from the syringe. Extrusion of a test droplet is recommended prior to entering eye, and excessive force on the plunger should be avoided.
Interactions:
There is an incompatibility between sodium hyaluronate and solutions containing quaternary ammonuiums, like benzalkonium chloride. because hyaluronan can precipitate in their presence. In that case Hyaclean shall not be in contact with medico chirurgical materials rinsed with such a solution or with ophthalmic topics containing a quaternary ammonium as a preserving agent.
Note:
The product is sterilized and free from pyrogenicity, and must be operated in a sterile environment;
It is for single use only. Do not use if sterile packaging has been damaged.
The used and expired products should be disposed in compliance with pertinent government regulations regarding medical devices;
The syringe is composed by glass. Be careful in using;
Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence;
Must not be used for intravenous injection;
The user must be trained to obtain relevant qualifications before using.
In case of any serious incident related to the device (especially adverse events), it shall be reported to the manufacturer or the EU authorised representative or the competent authority;
Pay attention to the physical injury caused by needles
Model:
15mg/mL: 0.5mL, 0.75mL, 1.0mL, 2.0mL
Shelf Life:
36 months
Storage :
Store between 2℃ and 20℃. do not freeze. Relative humidity no more than 80%, well ventilated and clean, no corrosive gas, protect from light. Do not injection after expiry date.
Issued date: 2024-05-23
Version number: 8.02.04.0340-A1