
UDEM Auditing: 11 July 2019 to 13 July 2019
As Singderm®, the second generation of modified hyaluronic acid and Surgiclean®, the oxidized regenerated cellulose for stopping bleed are applying for CE marking and are estimated to obtain it next year, UDEM, a notified body came to verify if the two products meet the specific technical requirements. This audit lasted for 3 days, which included
•Verifying the production process;
•Auditing quality management system;
•Reviewing the records of tests and machine maintenance
•Auditing contractors and checking the corresponding certifications
......
And we are happy to announce that the audit went smoothly. Singclean is highly praised for its comprehensive quality management system and quality control system.

Maestri Auditing: 14 July 2019 to 16 July 2019
Maestri is an third-party auditing body from Brazil, and this audit was performed by experienced auditors to evaluate if Singclean is in compliance with GMP standards. During the auditing, the auditors kept saying that Singclean was doing everything well from the files to production management and Singclean eventually passed the auditing without a single unqualified item.
The success of the two audits carried out within one week is due to Singclean’s strict quality control systems. Singclean always adheres to the principle: Quality First, Management Priority, Building Harmonious Enterprise, Creating Singclean Brand and Singclean is on the way to be the leading manufacturer of absorbable biomaterials in the world.
-
singclean | 2019-07-22
 2026 Singderm Global Academic Exchange Program First Stop Launches—— China-Thailand Aesthetic Injection Exchange& SymposiumFrom January 22nd to 23rd, Singderm successfully hosted the China-Thailand Aesthetic Injection Exchange& Symposium event, marking a significant step forward in promoting international collaboration within aesthetics. As a key strategic partner of Singderm in the Thai market, Pola Group invited a distinguished delegation of local experts, including: Dr. Rosalyn, Professor, Department of Anatomy, Dhurakij Pundit University, Bangkok Dr. Grace, Technical Director, Doctor Grace Clinic Dr. Jonny, Technical Director, Doctor Jonny Clinic The program was designed to deepen China-Thailand professional dialogue on injectable aesthetics and foster the integration and evolution of Eastern aesthetic concepts and techniques.
2026 Singderm Global Academic Exchange Program First Stop Launches—— China-Thailand Aesthetic Injection Exchange& SymposiumFrom January 22nd to 23rd, Singderm successfully hosted the China-Thailand Aesthetic Injection Exchange& Symposium event, marking a significant step forward in promoting international collaboration within aesthetics. As a key strategic partner of Singderm in the Thai market, Pola Group invited a distinguished delegation of local experts, including: Dr. Rosalyn, Professor, Department of Anatomy, Dhurakij Pundit University, Bangkok Dr. Grace, Technical Director, Doctor Grace Clinic Dr. Jonny, Technical Director, Doctor Jonny Clinic The program was designed to deepen China-Thailand professional dialogue on injectable aesthetics and foster the integration and evolution of Eastern aesthetic concepts and techniques. -
singclean | 2019-07-22
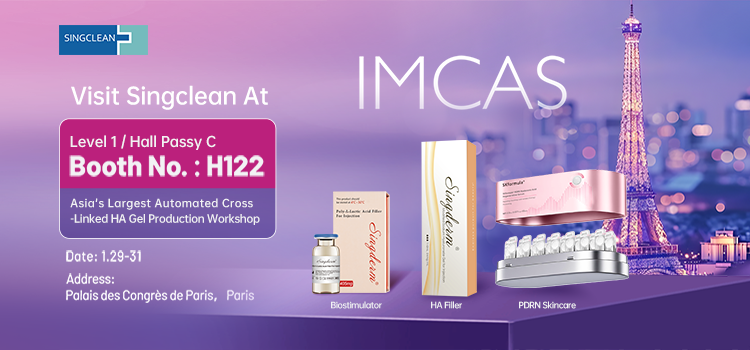 Singclean to Showcase Aesthetic Manufacturing Excellence and Singderm Brand Strength at IMCAS 2026
Singclean to Showcase Aesthetic Manufacturing Excellence and Singderm Brand Strength at IMCAS 2026 -
singclean | 2019-07-22
 Singclean Medical Strengthens Its Market Position at Russian Health Care Week 2025Singclean Medical successfully concluded its participation in Russian Health Care Week 2025, reinforcing its position as a comprehensive and reliable global supplier in the aesthetic and medical device industry. The exhibition served as a strategic platform for the company to showcase its diversified product portfolio, strong innovation capability, and growing influence in the Russian and neighboring markets.
Singclean Medical Strengthens Its Market Position at Russian Health Care Week 2025Singclean Medical successfully concluded its participation in Russian Health Care Week 2025, reinforcing its position as a comprehensive and reliable global supplier in the aesthetic and medical device industry. The exhibition served as a strategic platform for the company to showcase its diversified product portfolio, strong innovation capability, and growing influence in the Russian and neighboring markets.




