CE Singclean Monkeypox virus Antigen Test Kit
(Immunochromatography)
Instructions For Use
CE
Intended Use
Monkeypox virus Antigen Test Kit (Immunochromatography) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigen to Monkeypox virus in human body fluid. This test provides only a preliminary test result. Therefore, any reactive specimen with the Monkeypox virus Antigen Test Kit (Immunochromatography)must be confirmed with alternative testing method(s) and clinical findings.
Introduction
Monkeypox is a viral zoonosis (a virus transmitted to humans from animals) with symptoms very similar to those seen in the past in smallpox patients, although it is clinically less severe. With the eradication of smallpox in 1980 and subsequent cessation of smallpox vaccination, monkeypox has emerged as the most important orthopoxvirus for public health. Monkeypox primarily occurs in Central and West Africa, often in proximity to tropical rainforests and has been increasingly appearing in urban areas. Animal hosts include a range of rodents and non-human primates.
Principle
Monkeypox virus Antigen Test Kit (Immunochromatography) is a colloidal gold immunochromatographic assay.lt detects the virus Antigen of Monkeypox virus.
The test uses Monkeypox virus antibody (test line T) and goat anti-mouse IgG (control line C) immobilised on a nitrocellulose strip. The burgundy colored conjugate pad contains colloidal gold conjugated to another Monkeypox virus antibody conjugated with colloid gold and mouse IgG-gold conjugates. When the processed buffer containing the sample is added to the sample well, Monkeypox virus will combine with the Monkeypox virus antibody conjugate to form an antigen-antibody complex. This complex migrates through nitrocellulose membrane by capillary action. When the complex meets the line of the Monkeypox virus antibody of test line T, the complex is trapped forming a burgundy colored band which confirm a reactive test result. Absence of a colored band in the test region indicates a non-reactive test result.
The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex goat anti-mouse IgG/mouse IgG-gold conjugate regardless of the color development on any of the test bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
Materials Provided
Sealed pouches each containing a test cassette
A desiccant
Antigen extract buffer
Paper workbench (The small one-test-box can be used as a workbench)
Instruction for use
Materials Required But Not Provided
Timer
Specimen collection container
Packaging Specifications
1test/box,5tests/box,20tests/box
Precautions
· For in vitro diagnostic use only.
· Test is for single use only. Do not re-use under any circumstance.
· For laboratory professional use.
· Do not use after the expiration date indicated on the package. Do not use the test if its foil pouch is damaged. Do not reuse tests.
· All specimens should be considered potentially hazardous and handle in the same way as an infectious material.
· Avoid cross-contamination of specimens by using a new sponge collector for each specimen obtained.
· Read the entire procedure carefully prior to performing any tests.
· The used testing materials should be discarded in accordance with local, state and/or federal regulations.
· Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the Member State.
Storage And Stability
· Store in the sealed pouch at 4℃ to 30℃.
· The test is stable through the expiration date printed on the sealed pouch.
· The test must remain in the sealed pouch until use.
· After opening the sealed pouch, use the test as soon as possible within 60 minutes.
· Do not freeze.
· Do not use beyond the expiration date.
· The validity period is 24 months.
Specimen Collection
· Monkeypox virus Antigen Test Kit (Immunochromatography) can be performed using Body fluid samples.
· Samples collected in clean containers.
· Testing should be performed immediately after specimen collection.
· Bring specimens to room temperature prior to testing.
· If specimens are to be shipped, they should be packed in compliance with local regulations covering the transporation of etiologic agents.
Procedure
Allow the cube and specimen to equilibrate to room temperature (15-25°C) prior to 1.Remove the test cassette from the sealed foil pouch and use it as soon as possible.
2.Place the test device on a clean and level surface.
Test procedure:
1.Collection of human body fluid samples in antigen extraction tube.
2.Place the antigen extraction tube on the workbench.Place the antigen extraction buffer bottle vertically downward, squeeze the bottle to make the buffer drip freely into the extraction tube without touching the edge of the tube, and add 6 drops (about 200ul) to the extraction tube.
3.Install the dripper on the extraction tube and cap it tightly.
4.Open the aluminum foil bag and take out the test card, add 3 drops (about 100ul) into the sample hole of the test card (or use a pipette to add 100ul), and start the timer.
5.Wait for the colored line to appear. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
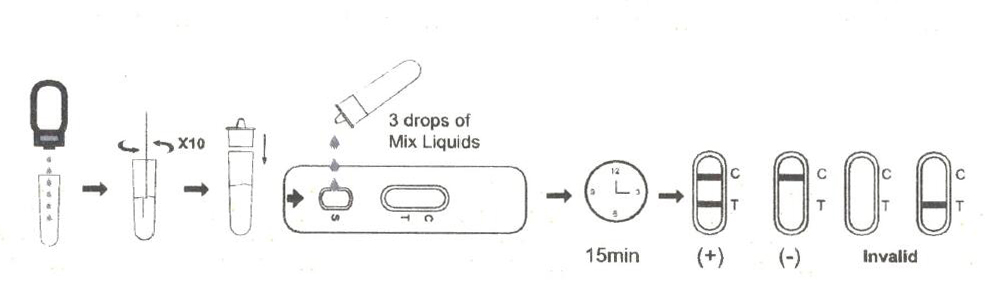
Interpretation Of Results
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no Monkeypox virus antigen is detected in the specimen. The result is negative.
Monkeypox virus positive:
In addition to the presence of C band, if T band is developed, the test indicates for the presence of Monkeypox virus antigen in the specimen. The result is Monkeypox virus positive.
INVALID:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Quality Control
Though there is an internal procedural control line in the test device of Control Region (C), the use of external controls is strongly recommended as good laboratory testing practice to confirm the test procedure and to verify proper test performance. Positive and negative control should give the expected results. When testing the positive and negative control, the same assay procedure should be adopted.
Limitations
1.Use fresh samples whenever possible.
2.Optimal assay performance requires strict adherence to the assay procedure described in this insert sheet. Deviations may lead to aberrant results.
3.A negative result for an individual subject indicates absence of detectable Monkeypox virus antigen. However, a negative test result does not preclude the possibility of exposure to or infection with Monkeypox virus.
4.A negative result can occur if the quantity of the Monkeypox virus antigen present in the specimen is below the detection limits of the assay, or failed to collect the Monkeypox virus antigen in human body fluid.
5.As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician after all clinical and laboratory findings have been evaluated.
6.Recent or remote vaccination with a vaccinia-based vaccine (e.g. anyone vaccinated before smallpox eradication, or more recently vaccinated due to higher risk such as orthopoxvirus laboratory personnel) might lead to false positive redults.
Latest Version: 8.74.04.004 A1 Issued Date: 2023.01.04