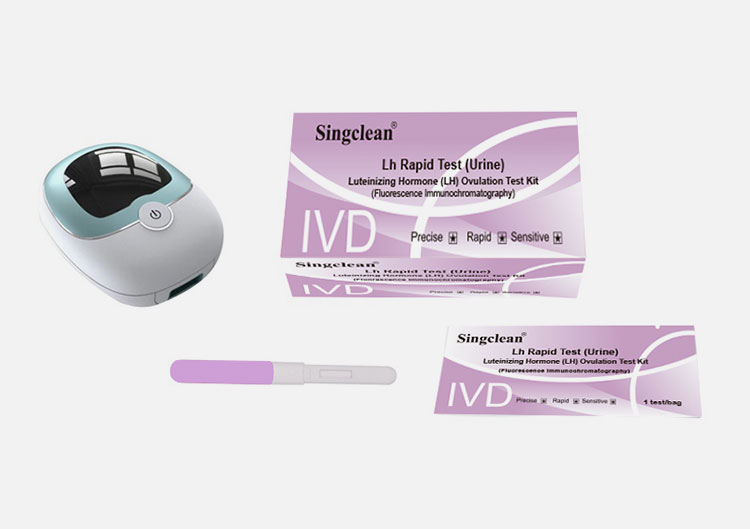
Luteinizing Hormone (LH) Test Kit
(Fluorescence Immunochromatography)
[Product name]
Luteinizing Hormone (LH) Test Kit (Fluorescence Immunochromatography)
[Packaging specifications]
10 tests/box, 20 tests/box, 25 tests/box, 30 tests/box, 50 tests/box, 100 tests/box.
[Intended use]
This kit is used for the quantitative determination of Luteinizing Hormone (LH) in human urine samples in vitro.
Human luteinizing hormone (LH) is a kind of glycoprotein hormones secreted by the pituitary gland, exists in human blood and urine, its role is to stimulate the release of the ovaries mature eggs. LH in the middle of the menstrual secretion, sharply form LH peak, from the basic level of 5 ~ 20 miu/ml rapidly rising to the peak of 25 ~ 200 miu/ml.Urine LH concentration is usually about 36 ~ 48 hours before ovulation suddenly jumped sharply, in 14 ~ 28 hours peak, peak about 14 to 28 hours after theca burst, mature eggs. LH within two days after ovulation back to the basic level, to enter the luteal phase, luteal phase usually lasts for 14 days. Then, the new follicles began to development, into the next menstrual cycle, so the cycle, until the pregnancy. Women in the LH peak within 1 ~ 3 days after the most fertile, thus, detection of LH in the urine levels can be used to predict ovulation time, can grasp the conception time effectively.
[Test principle]
This product uses the colloidal gold immune technology and the principle of double antibody sandwich method to qualitatively detect the LH presence level in female urine. A quality control line (line c) on that fib membrane in the reagent card is coated with goat anti-mouse IgG, a detection line (line t) is coat with an anti-LH monoclonal antibody -2, and the other end is coated with an anti-LH monoclonal antibody -1- colloidal gold complex. Dure detection, if LH is present in that urine sample, a complex is formed with the anti-LH monoclonal antibody -1 on colloidal gold and run on the membrane, and an antibody-antigen-antibody colloidal gold complex is formed with the anti-LH monoclonal antibody -2 on a detection line to produce a red line. On the other hand, the goat anti-mouse IgG on the control line was a polyclonal anti-antibody, which always combined with the antibody on the colloidal gold to form a red line with a certain depth, which was equivalent to the presence of 25mIU/mlLH as the contrast of the detection line. In the result judgment, if the intensity of the detection line was equal to or deeper than that of the control line, the result was positive. If the strength of the detection line is weaker than that of the control line or the wireless bar, the result is negative. In addition, a red line should always appear at line C, regardless of LH in the urine sample, indicating that the kit is valid and operating correctly.Principle of the supporting instrument: The measuring system of the instrument scans the binding area between the marker and the analyte on the test kit after the reaction to obtain the optical signal. Then the optical signal is measured and analyzed to quantitatively obtain the concentration of the analyte.
[Main components]
No. | Name | Component |
1 | Luteinizing Hormone (LH) Test Kit (Fluorescence Immunochromatography) | Each bag contains a test card and a desiccant; the test card consists of shell and a test strip, and the test strip consists of sample pad, fluorescent pad (fixed with fluorescently labeled LH monoclonal antibody 1), Nitrocellulose membrane (coated with LH monoclonal antibody 2 and goat anti-mouse IgG), filter paper and PVC plastic sheet |
2 | ID card | 1 pc/box |
[Applicable instrument]
Fluorescence immunoassay analyzer
[Storage conditions and validity period]
The test kit should be stored in a sealed aluminum foil bag at 4℃~30℃, and the validity period is 18 months. After the foil bag is opened, the validity period is 1 hour.
[Specimen Collectlon]
1. collect urine sample at 10:00 ~ 20:00 every day in order to get the best results. Do not collect urine urinated after waking up in the morning.
2. Liquid beverage should be restricted 2 hours, before collecting urine sample.
3. Use disposable plastic or glass container which is clean, dry and not contain any preservatives to collect fresh urine samples.
4. If not tested immediately, the urine samples can be stored at room temperature for 4 hours or at 2 ~8C for 12 hours, do not freeze,To achieve the best results, please take test on the day of urine collection. if the urine refrigerated, please standing at room temperature for about 30 minutes before the test.
5. If specimens are to be transported, they should be packaged in accordance with local regulations regarding the transport of pathogens.
[Test method]
Please read the instructions of the test kit and the manual of the fluorescence immunoanalyzer carefully before use.
a) Bring the test kit and sample to be tested to room temperature.
b) Make sure the ID card matches the batch number of the kit, and insert the ID card into the card reading area of the instrument to read the information.
c) Open the inner package of the test kit, take out the test card; draw 100μL of urine sample, drop vertically to the sampling place of the test card, and start timing;
d) After adding the sample, click "Start Test" on the screen of the fluorescence immunoanalyzer, and the test card will react at room temperature for 15 minutes; insert the test card into the test card slot of the fluorescence immunoanalyzer, and the instrument will automatically test the test card; The test results can be seen on the display screen of the fluorescence immunoanalyzer. Click "Print" on the screen to print the results.
[Positive judgment value or reference interval]
Normal reference value: <25mIU/mL
Due to differences in geography, race, gender and age, it is recommended that each laboratory establish its own positive judgment value or reference interval.
[Interpretation of test results] (For reference only, not used as clinical diagnostic criteria, test results need to be combined with other clinical and laboratory data for clinical diagnosis)
Content of LH (mIU/mL) | Clinical application advice |
Preovulation5-25 | Normal level |
Period of ovulation25-200 | LH is rapidly secreted in the middle of menstruation, and forms a LH peak, which rapidly increases from 5–20 mIU/mL in the basal level stage to 25–200 mIU/mL in the peak stage. The concentration of LH in urine suddenly and dramatically increases about 36–48 hours before ovulation, and peaks at 14–28 hours. The follicle membrane ruptures and mature eggs are excreted about 14–28 hours after the peak. |
[Quality Control]
Quality control should be carried out on a regular basis to ensure the validity and accuracy of test results. The test kit does not contain any quality control products, it is recommended to select appropriate quality control products for quality management.
[Limitations of the test method]
a) This test kit is only for the test of human urine samples. The test results need to be combined with other clinical and laboratory data, and if the LH test results do not match the clinical assessment, further testing is required.
b) False positive results may be caused by: cross-reaction of antibody-like components in the unrie.
c) False negative results may be caused by: some unknown components shield the antigenic determinants from binding to the antibody; the unstable LH antigen gradually degrades with time and temperature and cannot be recognized by the antibody. Effective test results depend on good reagent and sample storage environment.
d) Other factors, including technical reasons, operational errors and other sample factors, may also cause errors in test results.
[Product performance index]
a) Accuracy: The recovery rate is between 85% and 115%.
b) Linear range: within the linear range of 25~200mIU/ml, the linear correlation coefficient r ≥0.9900;
c) Blank limit: not higher than 25 mIU/ml;
d) Precision:
In-batch precision: The coefficient of variation (CV) is not more than 15%;
Precision between batches: The relative range between batches is not more than 15%.
[Precautions]
1) This product is a single-use in vitro diagnostic reagent. Do not use expired products.
2) The ID card and the test card cannot be used together, if they are different batches.
3) Please read the instructions of the test kit and the manual of the fluorescence immunoanalyzer carefully before use.
4) The environmental temperature should be 15℃~30℃ when testing. The test card stored at low temperature needs to be returned to room temperature before opening to avoid moisture absorption.
5) The test card and fluorescence immunoanalyzer should avoid vibration and electromagnetic environment when using. It is normal for the instrument itself to vibrate during normal use.
6) There is a desiccant in the aluminum foil bag, do not take it orally.
7) Appropriate biosafety assurance procedures should be in place for those substances containing or suspected of containing infectious agents. The following are the relevant precautions: Handle the sample with gloves or disinfect this reagent; disinfect spilled samples or reagents with disinfectant; disinfect and handle all specimens, reagents and potential contaminants in accordance with local regulations.
8) This product is used for rapid clinical diagnosis and screening, and the final diagnosis should be made by a doctor after comprehensive examination indicators and clinical symptoms.
9) The used material must be discarded immediately after test. Disposal should be in accordance with accepted medical practice and applicable national, local or institutional guidelines.
10) Do not use if package is open or damaged.