CE Singderm® Modified Sodium Hyaluronate Gel for Injection
CE 2292
Caution: General law restricts this device to sale by or on the order of a licensed physicianor properly licensed practitioner.
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
1. Device Description
Composition (every 1ml)
Sodium hyaluronate 24mg
Lidocaine hydrochloride 3mg
Phosphate buffer pH7.2 q.s.1ml
Singderm® is a sterile, biodegradable, nonpyrogenic, viscoelastic, clear, colorless, homogenized gel implant. It consists of modified hyaluronic acid (HA) produced by bacteria, formulated to a concentration of 24 mg/ml and 0.3% lidocaine in a physiologic buffer.
Singderm® is presented in a graduated, pre-filled, disposable syringe. Each box contains one syringe, an instruction leaflet and a set of labels in order to ensure traceability. The contents of the Singderm® syringe are sterilized by moist heat. If presense, the needles are sterilized by radiation or ethylene oxide.
2. Intended Use
Singderm® injectable gel implant is indicated for facial tissue augmentation by injection into areas in which restoration is required, including reconstructive treatment of volume loss as well as for facial morphological asymmetry.
3. Mode of Action
Singderm®is implanted to supplement the intercellular matrix and the intradermal tissue in order to restore lost anatomical structures. Its mechanism of action is based on the latest biotechnological developments in the production of injectable hyaluronic acid. The volume and the lifting capacity originate from the ability of hyaluronic acid to attract high amount of water, which is further increased by crosslinked process. The test resuts show that the product degraded a little at 12 weeks, degraded partly at 26 weeks, and almost degraded completely at 52 weeks.
4.CONTRAINDICATIONS
• Singderm® is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
• Singderm® contains trace amounts of gram-positive bacterial proteins and is contraindicated for patients with a history of allergies to such material.
• Singderm® contains trace amounts of lidocaine and is contraindicated for patients with a history of allergies to such material.
5.WARNINGS
• The product must not be injected into blood vessels. Introduction of Singderm®into the vasculature may occlude the vessels and could
cause infarction or embolization.
• Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or
infection is present should be deferred until the underlying process has been controlled.
6.PRECAUTIONS FOR USE
• Singderm® is packaged for single-patient use. Do not resterilize. Do not use if package is opened or damaged.
• Patients should be limited to 20 mL of Singderm®per 60 kg (130 lbs) body mass per year. The safety of injecting greater amounts has not been established.
• As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
• Singderm®is to be used as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, homogeneity, and performance of the product and it can therefore no longer be assured.
• The safety for use during pregnancy, in breastfeeding females, or in patients under 18 years has not been established.
• The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
• Singderm®should be used with caution in patients on immunosuppressive therapy.
• Patients who are using substances that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at injection sites.
• After use, treatment syringes and needles may be potential biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements.
• Singderm®injectable gel is a clear, colorless gel without particulates. In the event that the content of a syringe shows signs of separation and/or appears cloudy, do not use the syringe.
• If laser treatment, chemical peeling, or any other procedure based on active dermal response is considered after treatment with Singderm®, there is a possible risk of eliciting an inflammatory reaction at the indications site. An inflammatory reaction is also possible if the product is administered before the skin has healed completely after such a procedure.
• Failure to comply with the needle attachment instructions could result in needle disengagement and/or product leakage at the luer-lock and needle hub connection.
• If the needle is blocked, do not increase the pressure on the plunger rod but stop the injection and replace the needle.
• Athletes should be made aware that this product contains an active principle that may produce a positive result in anti-doping test.
• Medical practitioners must take into account the fact that this product contains lidocaine.
• The composition of this product is compatible with fields used for magnetic resonance imaging.
7.SIDE EFFECTS
The patients must be informed that they are potential side effects associated with implantation of this product, which may occur immediately or may be delayed. These include, but are not limited to:
• Inflammatory reactions(redness, oedema, erythema, etc.) which may be associated with itching or pain on pressure or both, occurring after the injection. These reactions may last for a week.
• Haematomas.
• Induration or nodules at the injection site.
• Staining or discolouration of the injection site.
• Poor effect or weak filling effect.
• Cases of necroses in the glabellar region, abscesses, granuloma and immediate or delayed hypersensitivity after hyaluronic acid injections have been reported. It is therefore advisable to take these potential risks into account.
• Patients must report inflammatory reactions which persist for more than one week, or any other side effect which develops, to their medical practitioner as soon as possible. The medical practitioner should use an appropriate treatment.
• Any other undesirable side effects associated with injection of Singderm® must be reported to the distributor and/or to the manufacturer.
8.METHOD OF USE & POSOLOGY
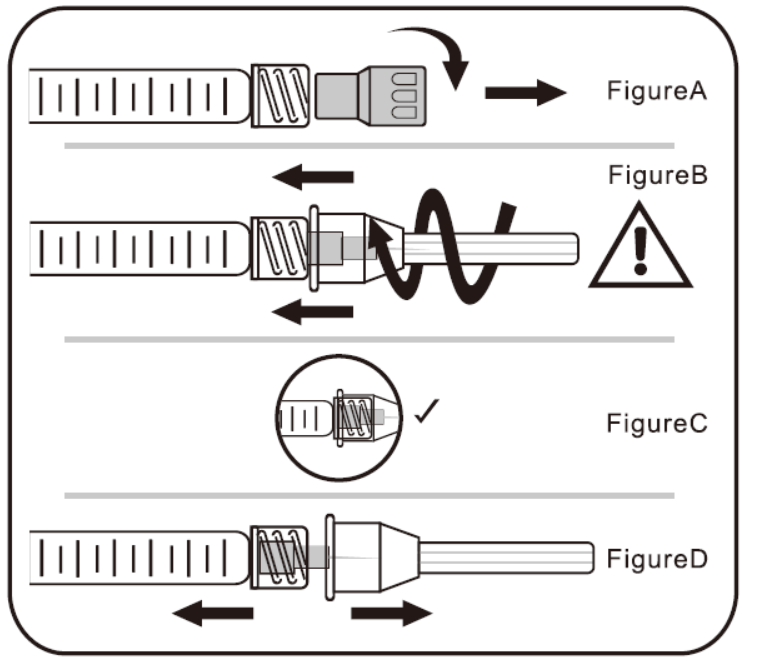
A. To Attach Needle to Syringe
STEP1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A
STEP 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle.
into the luer- lock end of the syringe.
STEP 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position as shown in Figure C.
NOTE: Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directionsto remove the needle cap as shown in Figure D
B. Physician Instructions
1) This product is designed to be injected into the dermis or the mucous membrane of the lips by an authorized medical practitioner in accordance with local applicable regulations. As precision is essential to a successful treatment, the product must be used by medical practitioners who have undertaken specific training in injection techniques for filling.
2) Before starting treatment patients should be informed of the product’s indications, contra-indications, incompatibilities and potential undesirable effects.
3) The area to be treated should be disinfected thoroughly prior to the injection.
4) Follow the above attaching needle to syringe steps, depress the plunger rod until the product flows out of the needle.
5) After the first small amount of material has been injected into the patient, wait a full 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection.
6) The injection technique may vary with regard to the angle and orientation of the bevel, the depth of injection, and the quantity administered. A linear threading technique, serial puncture injections, or a combination of the 2 have been used to achieve optimal results. Injecting the product too superficially may result in visible lumps and/or discoloration.
7) Inject Singderm® by applying even pressure on the plunger rod while slowly pulling the needle backward. The wrinkle should be lifted and eliminated by the end of the injection. It is important that the injection be stopped just before the needle is pulled out of the skin to prevent material from leaking out or ending up too superficially in the skin.
8) If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the injection and replace the needle.
9) The amount injected will depend on the areas which are to be corrected. Correct to 100% of the desired volume effect. Do not overcorrect. The degree and duration of the correction depend on the character of the defect treated, the tissue stress at the implant site, the depth of the implant in the tissue, and the injection technique.
10) When injection is completed, the treated site should be gently massaged so that it conforms to the contour of the surrounding tissues.
11) With patients who have localized swelling, the degree of correction is sometimes difficult to judge at the time of treatment. In these cases, it is better to invite the patient to a touch-up session after 1 to 2 weeks.
12) Patients may have mild to moderate injection-site responses, which typically resolve in a few days. If the treated area is swollen immediately after the injection, an ice pack can be applied to the site for a short period.
13) After the initial treatment, an additional treatment (from 1 to 2 weeks later) may be necessary to achieve the desired level of correction. If the wrinkle needs further treatment, the same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as wrinkle severity, skin elasticity, and dermal thickness at the treatment site.
14) The physician should instruct the patient to promptly report to her/him any evidence of problems possibly associated with the use of
Singderm®
9.Specifications
24mg/ml 1ml, 2ml,10ml.
10.Shelf Life and Storage
Shelf life is 2 years, Store at 2°C to 30°C, DO NOT FREEZE.
Fragile.
Latest version 8.12.04.302 A3 lssued date: 2022-04-24